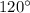Answer:
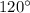
Step-by-step explanation:
In
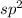 , hybridization, the hybridization of one s and one p takes place where one p orbital is unhybridized, the intermixing of the orbitals take place such that 3 identical hybrid orbitals having the same amount of energy are formed.
, hybridization, the hybridization of one s and one p takes place where one p orbital is unhybridized, the intermixing of the orbitals take place such that 3 identical hybrid orbitals having the same amount of energy are formed.
The electronic repulsion in between these hybrid orbitals is minimum and possess a trigonal planar geometry with the angle between the orbital being