Answer: The volume of oxygen gas required is 3.75 mL
Step-by-step explanation:
STP conditions:
1 mole of a gas occupies 22.4 L of volume.
We are given:
Volume of ammonia reacted = 3.00 mL = 0.003 L (Conversion factor: 1 L = 1000 mL)
The chemical equation for the reaction of ammonia with oxygen follows:
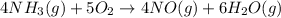
By Stoichiometry of the reaction:
(4 × 22.4) L of ammonia reacts with (5 × 22.4) L of oxygen gas
So, 0.003 L of ammonia will react with =
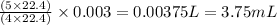 of oxygen gas
of oxygen gas
Hence, the volume of oxygen gas required is 3.75 mL