Answer:

Step-by-step explanation:
Equilibrium constant is defined as the ratio of concentration of products to the concentration of reactants each raised to the power their stoichiometric ratios. It is expressed as
 .
.
The given balanced equilibrium reaction is,
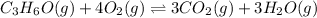
At eqm. conc. (0.51) M (0.30) M (1.8) M (2.0)M
The expression for equilibrium constant for this reaction will be,
![K_c=([CO_2]^3* [H_2O]^3)/([O_2]^4* [C_3H_6O]^1)](https://img.qammunity.org/2020/formulas/chemistry/college/83adkbf99xpdk3narq17jhcu0luhkiemt7.png)
Now put all the given values in this expression, we get :
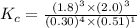
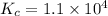
Thus the value of the equilibrium constant is
