Answer:
pKa of the histidine = 9.67
Step-by-step explanation:
The relation between standard Gibbs energy and equilibrium constant is shown below as:
![\Delta{G^0} =-RT \ln ([His])/([His+])](https://img.qammunity.org/2020/formulas/chemistry/high-school/1u9a15jvgixck4fy2h0e2g7h9apgha51a0.png)
R is Gas constant having value = 0.008314 kJ / K mol
Given temperature, T = 293 K
Given,
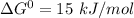
So, Applying in the equation as:-
![15\ kJ/mol=-0.008314\ kJ/Kmol* 293\ K* \ln ([His])/([His+])](https://img.qammunity.org/2020/formulas/chemistry/high-school/2ygs6x5fluluhibavxz8dx5le6qqnf2wq5.png)
Thus,
![15\ kJ/mol=-0.008314\ kJ/Kmol* 293\ K* \ln ([His])/([His+])](https://img.qammunity.org/2020/formulas/chemistry/high-school/2ygs6x5fluluhibavxz8dx5le6qqnf2wq5.png)
![([His])/([His+])=e^{(15)/(-0.008314* 293)](https://img.qammunity.org/2020/formulas/chemistry/high-school/3ocif7zao24ujg8xm2586axlltrecg0kur.png)
![([His])/([His+])=0.00211](https://img.qammunity.org/2020/formulas/chemistry/high-school/u87v89a6opbmh04l7or14mcqey4nrz9rj1.png)
Also, considering:-
![pH=pKa+log([His])/([His+])](https://img.qammunity.org/2020/formulas/chemistry/high-school/1tasacvh6jira8gry39830oqh4yj47mjtf.png)
Given that:- pH = 7.0
So,
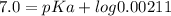
pKa of the histidine = 9.67