Answer:
20 drops of ethanol dispensed by dropper will be 1.0 mL ethanol.
Step-by-step explanation:
Mass of 15 drops of ethanol = 0.60 g
Mass of 1 drop of an ethanol =
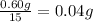
Mass of 1.0 mL of ethanol = m
Volume of ethanol = 1.0 mL
Density of an ethanol = d = 0.80 g/ml
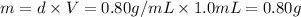
Number of drops in 0.80 g of ethanol = x
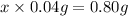
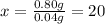
20 drops of ethanol dispensed by dropper will be 1.0 mL ethanol.