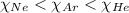Answer:
The order of mole fraction in increasing order will be:
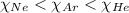
Step-by-step explanation:
Relation between mole fraction and partial pressure of the component is given as:
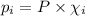
Where :P = Total pressure of the mixture
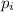 = partial pressure of the i component
= partial pressure of the i component
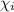 = Mole fraction of the i component
= Mole fraction of the i component
We have :
Partial pressure of the helium gas ,
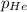 = 0.75 atm
= 0.75 atm
Partial pressure of the neon gas,
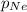 = 0.30 atm
= 0.30 atm
Partial pressure of the argon gas ,
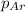 = 0.62 atm
= 0.62 atm
As we can see that from the above equation, that partial pressure and mole fraction are directly linked to each other.
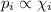
So, the gas with higher value of partial; pressure will have higher value of mole fraction in the mixture.
So, the order of partial pressure from lowest to highest is:
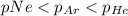
And hence the order of mole fraction will also be same :