Answer:
174.402 Torr is the partial pressure of oxygen gas.
Step-by-step explanation:
According to the Dalton's law, the total pressure of the gas is equal to the sum of the partial pressure of the mixture of gasses.
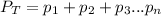
where,
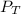 = Total pressure
= Total pressure
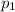 = partial pressure of gas-1
= partial pressure of gas-1
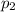 = partial pressure of gas-2
= partial pressure of gas-2
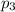 = partial pressure of gas-3
= partial pressure of gas-3
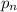 = partial pressure of nth gas in the mixture
= partial pressure of nth gas in the mixture
We have:
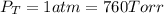
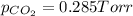
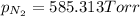
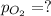
Now put all the given values is expression, we get the partial pressure of the
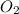 gas.
gas.
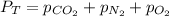
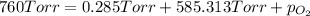
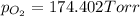
174.402 Torr is the partial pressure of oxygen gas.