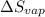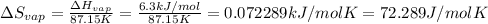Answer:
The entropy changes for the fusion and vaporization of argon is 15.634 J/mol K and 72.289 J/mol K respectively.
Step-by-step explanation:
The molar heats of fusion =
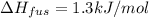
Melting point of argon = -190°C = 83.15 K
Entropy changes for the fusion =
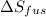
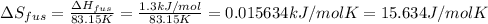
The molar heats of vaporization of argon =
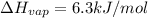
Boiling point of argon = -186°C = 87.15 K
Entropy changes for the vaporization=