Answer:
a. 0,903
b. 0,985
c. 0,996
Step-by-step explanation:
Partition coefficient between ether and water is:
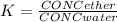
a. As k = 3,10, a single extraction with 300mL ether will give:
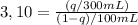
9,30 - 9,30q = q
9,30 = 10,30q
q = 0,903
b. Three extractions of 100 mL will extract:
First extraction:
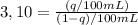
3,10 - 3,10q = q
3,10 = 4,10q
q = 0,756
The second extraction will 0,756 times of the solute that is in water. This solute is 1-0,756=0,244
Second extraction will extract 0,244*0,756 = 0,184
In the same way, third extraction will extraxt: (0,244-0,184)*0,756 = 0,045
Total solute extracted:
q = 0,756 + 0,184 + 0,045 = 0,985
c. Six extractions with 50mL of ether extract:
First extraction:
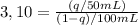
3,10 - 3,10q = 2q
3,10 = 5,10q
0,608 = q
Second extraction = (1-0,608)*0,608 = 0,234
Third extraction =(1-0,608-0,234)*0,608 = 0,096
Fourth extraction =(1-0,608-0,234-0,096)*0,608 = 0,038
Fifth extraction =(1-0,608-0,234-0,096-0,038)*0,608 = 0,015
Sixth extraction =(1-0,608-0,234-0,096-0,038-0,015)*0,608 = 0,005
q = 0,608+0,234+0,096+0,038+0,015+0,005 = 0,996
I hope it helps!