Answer: The mass of nitrogen gas reacted to produce given amount of energy is 5.99 grams.
Step-by-step explanation:
The given chemical reaction follows:
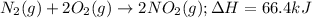
We know that:
Molar mass of nitrogen gas = 28 g/mol
We are given:
Enthalpy change of the reaction = 14.2 kJ
To calculate the mass of nitrogen gas reacted, we use unitary method:
When enthalpy change of the reaction is 66.4 kJ, the mass of nitrogen gas reacted is 28 grams.
So, when enthalpy change of the reaction is 14.2 kJ, the mass of nitrogen gas reacted will be =
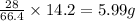
Hence, the mass of nitrogen gas reacted to produce given amount of energy is 5.99 grams.