Answer:
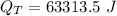
Step-by-step explanation:
Given:
- temperature of skin,
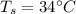
- initial temperature of steam vapour,
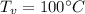
- latent heat of steam,
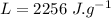
- mass of steam,
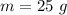
- specific heat of water,
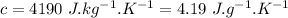
- final temperature,
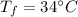
Assuming that no heat is lost in the surrounding.
We know:
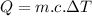
Now the total heat given by the steam to form water at the given conditions:
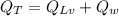 ..............................(1)
..............................(1)
where:
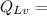 latent heat given out by vapour to form water of 100°C
latent heat given out by vapour to form water of 100°C
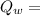 heat given by water of 100°C to come at 34°C.
heat given by water of 100°C to come at 34°C.
putting respective values in eq. (1)
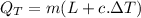
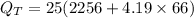
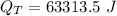
is the heat transferred to the skin.