Step-by-step explanation:
Net ionic equation is a chemical equation in which no spectator ions are written.
Complete ionic equation is a chemical equation in which spectator ions are written.
Molecular equation is a chemical equation in which molecuklar formula of compounds are but no ions .
a)
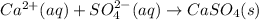
The given equation is net ionic equation as there are calcium ions and sulfate ions present in the chemical equation.
b)
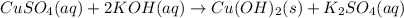
The given equation is a molecular equation as there are no ions present but only compounds on both reactant and product sides.
c)
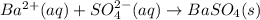
The given equation is net ionic equation as there are barium ions and sulfate ions present in the chemical equation.
d)
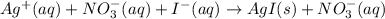
The given equation is complete ionic equation as there are silver ions, nitrate ions, and iodide ions present in the chemical equation.
e)
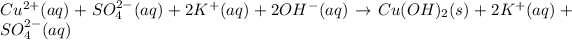
The given equation is complete ionic equation as there are copper ions,sulfate ions, potassium ions and hydroxide ions present in the chemical equation.
f)
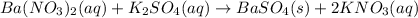
The given equation is a molecular equation as there are no ions present but only compounds on both reactant and product sides.