Answer:
1. Exothermic.
2. -1598 kJ.
Step-by-step explanation:
Hello!
1. In this case, according to the reaction, we can infer that 799 kJ of energy are evolved (given off, released) it means that the enthalpy of reaction is negative as the reactants have more energy than the products; which means this is an exothermic reaction.
2. Here, as we know that the enthalpy of reaction is -799 kJ/mol, we can compute the q-value as shown below, considering the reacted 2 moles of solid iron:
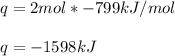
Which means that 1598 kJ of energy are evolved when 2 moles of solid iron react.
Best regards!