Answer : The percent by mass of the solution is 7.81 %
Explanation : Given,
Mass of NaCl = 8.75 g
Mass of solution = 112.0 g
Now we have to determine the percent by mass of the solution.
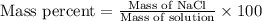
Putting values in above equation, we get:
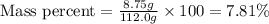
Therefore, the percent by mass of the solution is 7.81 %