Answer:
a. 34.69%
b. 20165.82 kJ/s
c. 12.4
Step-by-step explanation:
Assumptions:
1. Ideal rankine cycle
2. Isentropic stage 3-4
3. Isochoric process - constant volume
4. Isobutane is not a monoatomic gas, therefore cv is used for specific heat
a) The turbine stage is from 3 to 4
We know the inlet and outlet temperatures and pressures of the turbine. We can use butane thermodynamic tables to determine the enthalpies of stage 3 and stage 4
s₄=s₃
h₃=761.54kJ/kg
s₃=2.5457kg/kJ
P₄=410kPa
P₃=3250kPa
If it is isentropic whe can find the isentropic enthalpy by using the the entropy value of s₃ and the P₄:
h₄₍s₎ = 470.40 kJ/kg
Using the T₄:
h₄=689.74kJ/kg
The actual work of the turbine is defined as:
Wₐ = m(h₃-h₄)=m(761.54-689.74)=71.8m
Isentropic work = m (h₃-h₄₍s₎)=m(761.54-470.40)=290.84m
Isentropic work = actual work/isentropic work
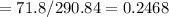
24.68
b)
To work out the total work output we need to determine the work of the pump.
We assume the Pressure is constant through the heat exchanger.
P₁=P₄
h₁ = 273.01 kJ/kg @ 410 kPa
v₁ = 0.001842 m³/kg
Wp,in = mv₁(P₂-P₁)/n
We determine the work of the pump:
Wp,in = 305.6*0.001842*(3250-410)/0.9=1776.26 kJ/s
Wout = Wp,in-Wt = 1776.26-305.6*71.8=20165.82 kJ/s out
c) Thermal efficiency is the Work out divided by the heat put in:
We have the water properties and have h₂ and h₃
Q = 162656 kJ/s
W = 20165.82 kJ/s
n = W/Q = 20165.82/162656=0.1240