Answer:
The molarity of I₃⁻ (aq) solution: M₂ = 0.186 M
Step-by-step explanation:
Given net ionic equation:
2S₂O₃²⁻ (aq) + I₃⁻ ( aq ) ⟶ S₄O₆²⁻ (aq) + 3I⁻ (aq)
Number of moles of S₂O₃²⁻: n₁ = 2, Number of moles of I₃⁻: n₂ = 1
Given- For S₂O₃²⁻ solution: Molarity: M₁ = 0.380 M, Volume: V₁ = 29.4 mL;
For I₃⁻ (aq) solution: Molarity: M₂ = ? M, Volume: V₂ = 30.0 mL
To calculate the molarity of I₃⁻ (aq) solution, we use the equation:
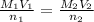
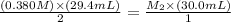
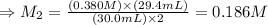
Therefore, the molarity of I₃⁻ (aq) solution: M₂ = 0.186 M