Answer:
a)
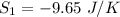
b)
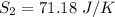
c) 0 J/K
d)S= 61.53 J/K
Step-by-step explanation:
Given that
T₁ = 745 K
T₂ = 101 K
Q= 7190 J
a)
The entropy change of reservoir 745 K
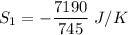
Negative sign because heat is leaving.
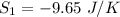
b)
The entropy change of reservoir 101 K
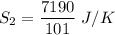
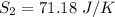
c)
The entropy change of the rod will be zero.
d)
The entropy change of the system
S= S₁ + S₂
S = 71.18 - 9.65 J/K
S= 61.53 J/K