Answer:
12.973 liters of H2S is required at STP.
Step-by-step explanation:
The given equation is ,
2H2S + 3O2 --> 2SO2 + 2H2O , mass of reacting O2 is 27.8 g
The mole ratio is,
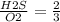
number of moles = m =
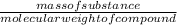
for O2, m =
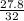 (molecular weight of O2 is 32)
(molecular weight of O2 is 32)
number of moles of H2S is
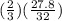
now at STP, 1 mole of gas occupies 22.4 liters.
thus liters of H2S required are,
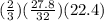 = 12.973 liters
= 12.973 liters