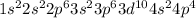Answer : The electronic configuration of selenium (Se) is:
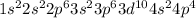
Step-by-step explanation:
Electronic configuration : It is defined as the representation of electrons around the nucleus of an atom.
Number of electrons in an atom are determined by the electronic configuration.
As we know that selenium is the non-metal that belongs to group 16 and has 34 electrons in their shell.
The electronic configuration of selenium (Se) will be: