To solve this problem it is necessary to apply the concepts related to the work done in an isobaric system, that is, where the pressure is constant.
The mathematical equation that can define this process is given by
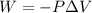
Where
P = Pressure
V = Change in Volume
Our values are given as
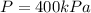
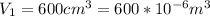
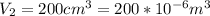
Replacing we can find the total work made,
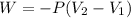
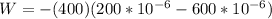
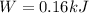
The change of sign is indicating the work done in the gas.
By the first law of thermodynamics we can now calculate the total internal energy where
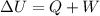
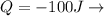 Heat energy TRANSFERRED OUT of the gas
Heat energy TRANSFERRED OUT of the gas
Replacing
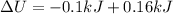
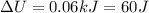
Therefore the change in thermal energy of gas during this process is 60J