Answer:
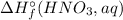 = (-207.39) kJ/mol
= (-207.39) kJ/mol
Step-by-step explanation:
Given:
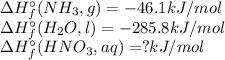
3NO₂(g)+H₂O(l)→ 2HNO₃(aq)+NO(g); ΔH° = -137.3 kJ ....equation 1
2NO(g)+O₂(g)→ 2NO₂(g) ; ΔH° = -116.2 kJ ....equation 2
4NH₃(g)+5O₂(g)→ 4NO(g)+6H₂O(l) ;ΔH° = -1165.2 kJ ....equation 3
Multiplying equation 1 with (2/3), we get
2NO₂(g) + 2/3 H₂O(l) → 4/3 HNO₃(aq) + (2/3) NO(g) ....equation 4
Adding equation 2 and 4, we get
2NO₂(g) + 2/3 H₂O(l) + 2NO(g) + O₂(g) → 4/3 HNO₃(aq) + (2/3) NO(g) + 2NO₂(g)
⇒ (4/3) NO(g) + O₂(g) + 2/3 H₂O(l) → 4/3 HNO₃(aq) ....equation 5
Multiplying equation 3 with (1/3), we get
(4/3) NH₃(g) + (5/3) O₂(g) → (4/3) NO(g)+ 2 H₂O(l) ....equation 6
Now adding equation 5 and 6, we get
(4/3) NH₃(g) + (5/3) O₂(g) + (4/3) NO(g) + O₂(g) + 2/3 H₂O(l) → (4/3) NO(g)+ 2 H₂O(l) + 4/3 HNO₃(aq)
⇒ (4/3) NH₃(g) + (8/3) O₂(g) → (4/3) HNO₃(aq) + (4/3) H₂O(l) ....equation 7
Now multiplying equation 7 with (3/4), we get
NH₃(g) + 2 O₂(g) → HNO₃(aq) + H₂O(l) ...equation 8
Therefore, by Hess's law the standard enthalpy of formation is:
ΔH° = (3/4) [ (-137.3 kJ) × (2/3) + (-116.2 kJ) + (-1165.2 kJ) × (1/3)]
ΔH° = (3/4) [ - 91.53 - 116.2 - 388.4]
ΔH° = (3/4) [-596.13] = -447.09 kJ
Since the change in enthalpy of a reaction:
ΔH° =
![[\Delta H_(f)^(\circ ) (HNO_(3), aq) + \Delta H_(f)^(\circ ) (H_(2)O, l)] - [\Delta H_(f)^(\circ ) (NH_(3), g) + 2 \Delta H_(f)^(\circ ) (O_(2), g)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/xcouk5r5b8qs82nuj7klvwhk12gluzfdqh.png)
(-447.09 kJ) =
 + (-285.8 kJ)] - [-46.1 kJ + 2 (0 kJ)]
+ (-285.8 kJ)] - [-46.1 kJ + 2 (0 kJ)]
⇒
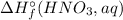 = (-447.09) + 285.8 kJ - 46.1 kJ
= (-447.09) + 285.8 kJ - 46.1 kJ
⇒
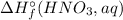 = (-207.39) kJ/mol
= (-207.39) kJ/mol