Answer:
The enthalpy of hydration of copper sulphate is -1486.62 kJ/mol which means 1486.62kJ of energy is absorbed by one mole of copper sulphate during the process of hydration
Step-by-step explanation:
Step 1: Determine the energy released per mole of
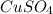 dissolved
dissolved
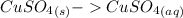 (Eq. 1)
(Eq. 1)
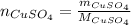
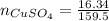
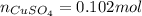
If 0.102 moles of
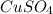 releases 55.51kJ of energy, 1 mole will release 541.85kJ/mol
releases 55.51kJ of energy, 1 mole will release 541.85kJ/mol
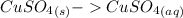 ΔH = -541.85kJ/mol
ΔH = -541.85kJ/mol
Step 2: Determine the energy released per mole of
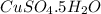 dissolved
dissolved
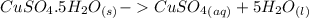 (Eq. 2)
(Eq. 2)
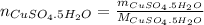
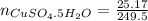
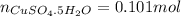
If 0.101 moles of
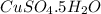 absorbs 95.31kJ of energy, 1 mole will absorb 944.77kJ/mol
absorbs 95.31kJ of energy, 1 mole will absorb 944.77kJ/mol
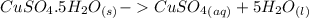 ΔH = 944.77kJ/mol
ΔH = 944.77kJ/mol
Step 3: Subtracting Eq. 2 from Eq. 1
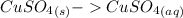 ΔH = -541.85kJ/mol (Eq. 1)
ΔH = -541.85kJ/mol (Eq. 1)
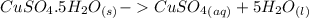 ΔH = 944.77kJ/mol (Eq. 2)
ΔH = 944.77kJ/mol (Eq. 2)
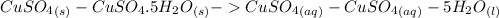 ΔH = -541.85-944.77
ΔH = -541.85-944.77
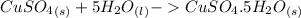 ΔH = -1486.62 kJ/mol
ΔH = -1486.62 kJ/mol