Answer:
288.2 K
Step-by-step explanation:
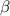 = Volumetric expansion coefficient =
= Volumetric expansion coefficient =
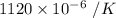
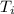 = Initial temperature = 273.1 K
= Initial temperature = 273.1 K
 = Final temperature
= Final temperature
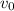 = Original volume = 150 mL
= Original volume = 150 mL
Change in volume is given by
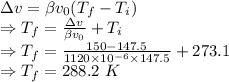
The temperature of the ethyl alcohol should be 288.2 K to reach 150 mL