Answer:
11·699
Step-by-step explanation:
Given the concentration of hydroxide ion in the solution is 5 ×
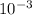 M
M
Assuming the temperature at which it is asked to find the pH of the solution be 298 K
At 298 K the dissociation constant of water is
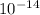
∴ pH + pOH = 14 at 298 K
pOH of the solution = -log( concentration of hydroxide ion )
∴ pOH of the given solution = - log(5 ×
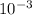 = -0·699 + 3 = 2·301
= -0·699 + 3 = 2·301
pH of the given solution = 14 - 2·301 = 11·699
∴ pH of the solution = 11·699