Answer:
0,508g of H₂O₂
Step-by-step explanation:
For the reaction:
2KMnO₄(aq) + H₂O₂(aq) + 3H₂SO₄(aq) → 3O₂(g) + 2MnSO₄(aq) + K₂SO₄(aq) + 4H₂O(l)
2 moles of KMnO₄ react with 1 mol of H₂O₂.
In the titration, moles of KMnO₄ required were:
1,68M×0,0178L = 0,0299 moles of KMnO₄. Moles of H₂O₂ are:
0,0299 moles of KMnO₄×
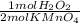 = 0,01495 moles of H₂O₂. As molar mass of H₂O₂ is 34,01g/mol, mass of H₂O₂ was dissolved is:
= 0,01495 moles of H₂O₂. As molar mass of H₂O₂ is 34,01g/mol, mass of H₂O₂ was dissolved is:
0,01495 moles of H₂O₂×
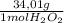 = 0,508g of H₂O₂
= 0,508g of H₂O₂