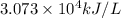Answer : The fuel value and the fuel density of pentane is, 49.09 kJ/g and
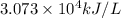 respectively.
respectively.
Explanation :
Fuel value : It is defined as the amount of energy released from the combustion of hydrocarbon fuels. The fuel value always in positive and in kilojoule per gram (kJ/g).
As we are given that:
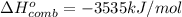
Fuel value =
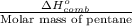
Molar mass of pentane = 72 g/mol
Fuel value =
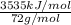
Fuel value = 49.09 kJ/g
Now we have to calculate the fuel density of pentane.
Fuel density = Fuel value × Density
Fuel density = (49.09 kJ/g) × (0.626g/mL)
Fuel density = 30.73 kJ/mL =
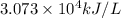
Thus, the fuel density of pentane is