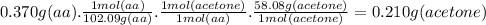Answer:
0.210 g
Step-by-step explanation:
Let's consider the following balanced equation.
CH₃C(O)CH₂CO₂H → CH₃C(O)CH₃ + CO
We can establish the following relations:
- The molar mass of acetoacetic acid is 102.09 g/mol
- The molar ratio of CH₃C(O)CH₂CO₂H to CH₃C(O)CH₃ is 1:1.
- The molar mass of acetone is 58.08 g/mol.
The mass of acetone produced from 370 mg (0.370 g) of acetoacetic acid (aa) is: