Answer:
Percentage by mass of calcium carbonate in the sample is 93.58%.
Step-by-step explanation:
Assumptions:
- calcium carbonate was dissolved completely and the amount of carbon dioxide released was proportional to the amount of calcium carbonate.
- the sample did not contain any other compound that released or reacted with carbon dioxide.
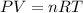
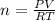
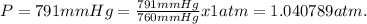
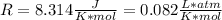
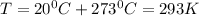
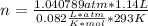
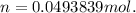
given the equation of the reaction:
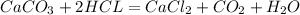
from assumption:
amount carbon dioxide = amount of calcium carbonate
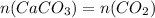
reacting mass ( m ) = Molar Mass ( M ) * Amount ( n )
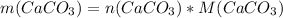
m = 0.04938397
 * 100
* 100
 = 4.93839
= 4.93839
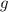
percentage by mass of CaC
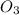 =
=
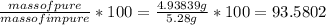 .
.