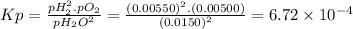Answer:
6.72 × 10⁻⁴
Step-by-step explanation:
Let's consider the following elementary reaction.
2 H₂O(g) ⇄ 2 H₂(g) + O₂(g)
The equilibrium constant (Kp) is the product of the partial pressures of the products raised to their stoichiometric coefficients divided by the product of the partial pressures of the reactants raised to their stoichiometric coefficients.