Answer : The product of this second decay reaction is, polonium (Po).
Explanation :
Alpha decay : In this process, alpha particles is emitted when a heavier nuclei decays into lighter nuclei. The alpha particle released has a charge of +2 units.
The general representation of alpha decay reaction is:
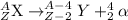
When radium undergoes alpha decay, the alpha decay reaction will be,
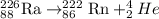
When product (radon) undergoes alpha decay, the alpha decay reaction will be,
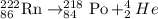
So, the element is polonium (Po) that has atomic number 84 and atomic mass 218.
Hence, the product of this second decay reaction is, polonium (Po).