Answer:
Van der Waal's equation
Step-by-step explanation:
The Van der Waal's equation is use to calculate the properties of a gas under nonideal or real gases conditions.
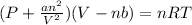 .
.
Here P, V ,T ,n and R have usual meaning as in the ideal gas equation
that is PV=nRT
with the difference of constant a and b. a and b are constants representing magnitude of intermolecular attraction and excluded volume respectively respectively.