Answer : The expected coordination number of NaBr is, 6.
Explanation :
Cation-anion radius ratio : It is defined as the ratio of the ionic radius of the cation to the ionic radius of the anion in a cation-anion compound.
This is represented by,
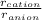
When the radius ratio is greater than 0.155, then the compound will be stable.
Now we have to determine the radius ration for NaBr.
Given:
Radius of cation,
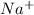 = 102 pm
= 102 pm
Radius of cation,
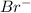 = 196 pm
= 196 pm
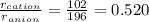
As per question, the radius of cation-anion ratio is between 0.414-0.732. So, the coordination number of NaBr will be, 6.
The relation between radius ratio and coordination number are shown below.
Therefore, the expected coordination number of NaBr is, 6.