Answer:
Avogadro’s number was calculated by determining the number of atoms in 12.00 g of carbon-12.
Step-by-step explanation:
The number of particles presents in one mole of a substance is known as Avogadro's number.
Avogadro's number is
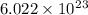 atoms or molecules or ions or particles present in one mole of a substance. It is denoted by the symbol
atoms or molecules or ions or particles present in one mole of a substance. It is denoted by the symbol
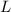 or
or
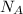 . It is a dimensionless quantity.
. It is a dimensionless quantity.
Avogadro's number was proposed by Jean Perrin but named in the honor of italian scientist Amedeo Avogadro.
Avogadro's number is the number of atoms present in 12 grams of carbon-12.