Answer:
The ice will start to melt after 25.4 mins.
Step-by-step explanation:
To melt the ice , heat should be supplied such that two processes take place-
i) Increasing the temperature of ice upto 0.0°C
ii) Phase change from ice to water at constant temperature of 0.0°C
Given that,
Initial temperature
 = -25.0°C
= -25.0°C
Final temperature
 = 0.0°C
= 0.0°C
mass of ice , m = 456 g = 0.456 kg
Specific heat of ice , s = 2090 J/(kg K)
Latent heat of fusion , L = 33.5 × 104 J/kg
For process i) , heat that should be supplied is -
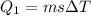 =
=
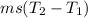 = 0.456×2090×(0-(-25))
= 0.456×2090×(0-(-25))
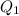 = 23826 J
= 23826 J
For process ii) , heat that should be supplied is -
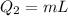 = 0.456×33.5 × 104 = 1588.704 J
= 0.456×33.5 × 104 = 1588.704 J
∴Total heat required is -
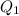 +
+
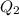 = 25414.704 J
= 25414.704 J
Rate of heat supply (r) = 1000 J/min
∴ The required time (t) is given by -
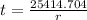 =
=
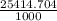 = 25.4 mins
= 25.4 mins
∴ t = 25.4 mins