Answer:
The hydrogen ion concentration is
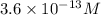 .
.
Step-by-step explanation:
From the given,
Volume of NaOH = 500 ml = 0.500L
Moarity of NaOH = 0.156M
The number of moles of NaOH is calculated is a follows.
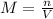
Substitute the given values.
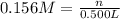
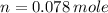
Volume of
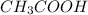 = 500 ml = 0.500L
= 500 ml = 0.500L
Moarity of
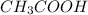 = 0.100M
= 0.100M
The number of moles of
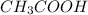 is calculated is a follows.
is calculated is a follows.
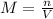
Substitute the given values.
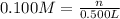
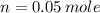
NaOH is a strong electrolyte
The number of moles NaOH left in the solution is
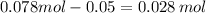
Therefore, hydroxide ion concentration is 0.028 mol
The hydrogen concentration
![[H^(+)][OH^(-)]=10^(-14)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/dctdvqy78b4qxvk9n2zfmcrab6yx6lxgmz.png)
![[H^(+)]= \frac {10^(-14)}{[OH^(-)]}](https://img.qammunity.org/2020/formulas/chemistry/middle-school/l0w4repyw5o9q2uta8sar8is9yi0puwejk.png)
![[H^(+)]= \frac {10^(-14)}{0.028}=3.6 * 10^(-14)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/a06g65sdcy5pwzwexpvmpsqd4itxtdq35s.png)
Therefore, hydrogen ion concentration is
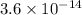 ,
,