Answer:
The maximum wavelength of light for which a carbon-carbon triple bond could be broken by absorbing a single photon is 143 nm.
Step-by-step explanation:
It takes 839 kJ/mol to break a carbon-carbon triple bond.
Energy required to break 1 mole of carbon-carbon triple bond = E = 839 kJ
E = 839 kJ/mol = 839,000 J/mol
Energy required to break 1 carbon-carbon triple bond = E'
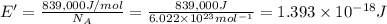
The energy require to single carbon-carbon triple bond will corresponds to wavelength which is required to break the bond.
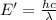 (Using planks equation)
(Using planks equation)
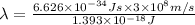
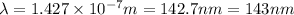
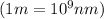
The maximum wavelength of light for which a carbon-carbon triple bond could be broken by absorbing a single photon is 143 nm.