Answer:
There are approximately 1.54 moles in a 275 g sample of
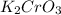 .
.
Step-by-step explanation:
To find out number of moles, fistly we have to calculate molecular mass of
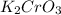 .
.
There are 2 atoms of Potassium 1 atom of Chromium and 3 atoms of oxygen in the given compound.
For molecular mass we have to add the value of mass of 2 atoms of Potassium with mass of 1 atom of Chromium and with mass of 3 atoms of oxygen.
Atomic mass of Potassium = 39
Atomic mass of Chromium = 52
Atomic mass of Oxygen = 16
Now,
Molecular mass of
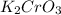 =
=
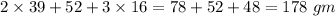
The molecular mass of a compound is the mass of compound in one mole.
To find out the number of moles, we have to divide given mass of compound by its molecular mass.
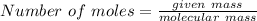
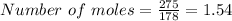
Hence the number of moles in 275 gm of
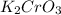 is 1.54.
is 1.54.