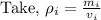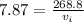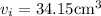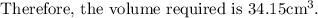Answer:
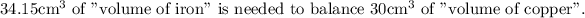
Step-by-step explanation:
Density of an object is defined as the mass occupied per unit volume. “Density” is represented by “ρ”. Mathematically,
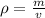 where, “m" mass of an object and "v" is volume of an object.
where, “m" mass of an object and "v" is volume of an object.
Given that,
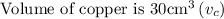
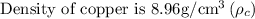
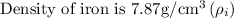
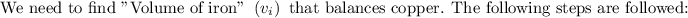
Step: 1
To find the mass of the copper:
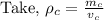
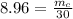
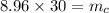
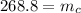
Mass of the copper is 268.8 kg.
To balance the weight of the copper 268.8 kg
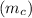 = weight of the iron
= weight of the iron
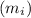 the volume required is
the volume required is
Step: 2