Answer:
In thioformaldehyde molecule,
Central atom - C
lone pairs on central atom - zero
Ideal bond angle between the carbon -hydrogen bonds -
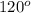
Compared to the ideal angle, you would expect the actual angle between the carbon-hydrogen bonds to be
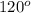 .
.
Step-by-step explanation:
The structure of thioformaldehyde is as follows. (in attachment).
From the structure,
Central atom - C
There is no lone pairs are present on the carbon atom, Hence,lone pairs on central atom - zero
The geometry of thioformaldehyde is Trigonal planar.
Hence, the ideal bond angle is
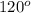 .
.
Ideal bond angle between the carbon -hydrogen bonds -
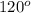
All bonds in the thioformaldehyde is similar. Therefore,actual angle between the carbon-hydrogen to be
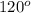 .
.