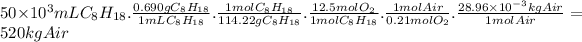Answer:
520 kg
Step-by-step explanation:
Let's consider the combustion of isooctane.
C₈H₁₈(l) + 12.5 O₂(g) → 8 CO₂(g) + 9 H₂O(l)
We can establish the following relations:
- 1 mL of C₈H₁₈ has a mass of 0.690 g (ρ = 0.690 g/mL).
- The molar mass of C₈H₁₈ is 114.22 g/mol.
- The molar ratio of C₈H₁₈ to O₂ is 1:12.5.
- The mole fraction of O₂ in air is 0.21.
- The molar mass of air is 28.96 g/mol.
50 L of isooctane require the following mass of air.