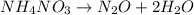Answer:
The correct answer is : No, because there are 4 hydrogen atoms on the reactants side and 2 on the products side.
Step-by-step explanation:
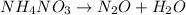
The given reaction equation is not balanced because:
- Number of hydrogen atoms on both sides are not equal that is 4 on reactants side and 2 on products side.
- Number of oxygen atoms on both sides are not equal that is 3 on reactants side and 2 on products side.
In a balanced chemical equation number of atoms of each elements are equal on both sides.
So, the balanced chemical equation will be: