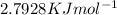Answer:
Latent Heat of Vaporization of Nitrogen is
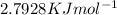
Step-by-step explanation:
• We have learned that adding thermal energy by heat increases the temperature of a substance.
• But surprisingly, there are situations where adding energy does not change the temperature of a substance at all. Instead, the additional thermal energy acts to loosen bonds between molecules or atoms and causes a phase change.
• Because this energy enters or leaves a system during a phase change without causing a temperature change in the system, it is known as latent heat (latent means hidden).
• Latent heat of vaporization is a physical property of a substance.
• It is defined as the heat required to change one mole of liquid at its boiling point under standard atmospheric pressure. It is expressed as kg/mol or kJ/kg.
• When a material in liquid state is given energy, it changes its phase from liquid to vapor; the energy absorbed in this process is called heat of vaporization.
• Latent Heat of Vaporization of Nitrogen is