Answer:
Oxidized and reducing agent: chromium.
Reduced and oxidizing agent: mercury.
Step-by-step explanation:
Hello!
In this case, for the reaction:
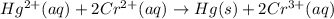
We keep in mind that the species that increase the oxidation state is the oxidized one whereas the one that decrease the oxidation state is the reduced one; therefore chromium is the oxidized one as well as the reducing agent because it goes from +2 to +3 and mercury the reduced one as well as the oxidizing agent because it goes from 2+ to 0.
Best regards