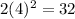Answer:First energy level = 2electrons
Second energy level = 8 electrons
Third energy level = 18 electrons
Fourth energy level = 32 electrons
Explanation: Each energy level can contain up to
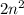 electrons. Where n indicates number of level
electrons. Where n indicates number of level
For first energy level where n=1
Number of electrons =
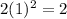
For second energy level where n =2 Number of electrons =
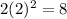
For Third energy level where n=3
Number of electrons=
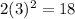
For fourth energy level where n=4
Number of electrons =