Answer:
First-order with respect to hydrogen.
Step-by-step explanation:
Hello!
In this case, considering that the rate law of this reaction can be expressed via:
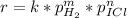
If we want to know m, the order of reaction with respect to hydrogen, we need to relate the experiments 1 and 3 in order to get rid of the pressure of ICl:
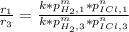
Thus, we plug in the given rates, and pressures to get:
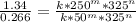
So we can cancel wout k and 325^n:
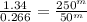
Next we solve for m, the order of reaction with respect to hydrogen:
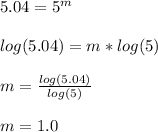
It means it is first-order with respect to hydrogen.
Regards!