Answer:
0.800 moles of
 will be produced when 0.400 moles of
will be produced when 0.400 moles of
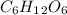 react and are decomposed
react and are decomposed
Step-by-step explanation:
The decomposition of
![C_6H_1_2O_6[/te can be written as </p><p>[tex]C_6H_1_2O_6](https://img.qammunity.org/2020/formulas/chemistry/middle-school/4mgcvjsqmmnxiawh4fq2ixbrk4yghgm3mx.png) (aq) ------> 2C2H5OH (aq) + 2CO2
(aq) ------> 2C2H5OH (aq) + 2CO2
¬moles of
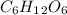 = 0.400 moles
= 0.400 moles
Moles of
 produced will be calculated by the formula,
produced will be calculated by the formula,
Moles of CO2 = 0.400 moles of
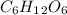
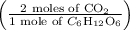
Substituting the values,
Moles of
 =
=
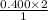 = 0.400*2 = 0.800 moles
= 0.400*2 = 0.800 moles
Therefore, 0.800 moles of
 will be produced when 0.400 moles of
will be produced when 0.400 moles of
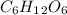 react and are decomposed
react and are decomposed