Answer:
Theoretical yield is 0,041g of caffeine
Step-by-step explanation:
The partition coefficient formula is:

Where k is partition coefficient (4,6)
The Caffeine in water at equilibrium will have a concentration of:
(0,10g-x) / 100mL
And Caffeine in dichloromethane will be:
xg / 15mL -Where x grams are the theoretical yield of caffeine-
Replacing:
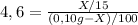
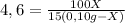
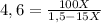
6,9 - 69X = 100X
6,9 = 169X
0,041 g = X
I hope it helps!