Answer:
The catalyzed reaction will take 1,41 s
Step-by-step explanation:
The rate constant for a reaction is:
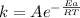
Assuming frequency factor is the same for both reactions (with and without catalyst) it is possible to obtain:
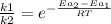
Replacing:
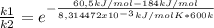
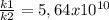
That means the reaction occurs 5,64x10¹⁰ faster than the uncatalyzed reaction, that is 2537 years / 5,64x10¹⁰ = 4,50x10⁻⁸ years. In seconds:
4,50x10⁻⁸ years×
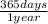 ×
×
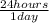 ×
×
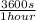 = 1,41 s
= 1,41 s
I hope it helps!