Answer:
538L of carbon dioxide will be produced
Step-by-step explanation:
This is a classic example of combustion of an alkane.
If balanced equation is not given; this format can come in handy.
For any alkane of the type : CₙH₂ₙ₊₂ , it's combustion reaction will follow:
2CₙH₂ₙ₊₂ + (3n+1) O₂ → (2n)CO₂ + 2(n+1) H₂O
For ethane:
2C₂H₆(g) + 7O₂(g) → 4CO₂(g) + 6H₂O(l)
Here, 2 mole of ethane gives 4 moles of CO₂.
or,
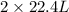 of ethane gives
of ethane gives
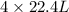 of CO₂.
of CO₂.
Then, 269L of ethane will give:
=
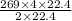
=
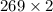 L
L
= 538 L
Therefore, 538L of carbon dioxide will be produced