Answer:
Lewis structure for nitrogen triiodide,
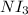 is given in the attachment.
is given in the attachment.
Step-by-step explanation:
Given:
The given compound is Nitrogen triiodide. In which 1 atom of Nitrogen combines with 3 atoms of Iodine. Both Nitrogen and Iodine are non-metals,So they form covalent bond by sharing of electrons.
The electron configuration of Nitrogen and Iodine is given below;
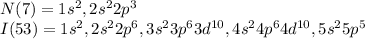
There are 5 electrons in valance shell of Nitrogen atom and 7 electrons in valance shell of Iodine atom.
So, 3 atom of Iodine shares 1 electron with 1 electrons of Nitrogen.
The Lewis dot Structure is in the attachment.